Visualizing regulatory procedure: mastering the MAA process with BPMN
Written by Andrea
19 January 2026 · 10 min read

Launching a new drug is a massive milestone in the pharmaceutical product lifecycle. Yet, it is also a massive orchestration of global regulatory standards, diverse stakeholders both within the company and among approval entities, and extensive documentation to prepare and revise.
The European Medicines Agency (EMA) drug approval process, specifically the Marketing Authorisation Application (MAA), is the gateway for pharmaceutical companies to the European Economic Area (EEA), allowing them to obtain a single authorisation to market a new drug that is valid across the entire European Union. However, when we look at it more closely, it becomes clear that the path is rarely a straight line. Because it involves rigorous scientific examination and multiple entities such as the EMA, Committee for Medicinal Products for Human Use (CHMP), and the European Commission, the MAA phases can feel fragmented and overwhelming.
While the procedure is demanding and lengthy, it is also standardized. This makes it highly predictable. Whether your new drug reaches the market doesn’t rest on clinical data alone. It also relies on how you manage the flow of information between your team and the evaluation committees.
In an environment defined by strict legal timeframes and "clock stops”, on-point communication is the difference between a drug reaching patients or stalling in the pipeline.
By stripping these procedural mechanics to the bone—turning tasks, timelines, and dependencies into visual diagrams—you can transform a fragmented regulatory hurdle into an optimized strategy for market access. This is where BPMN comes in handy.
Why use BPMN for regulatory affairs? The strategic advantage
To master the EMA drug approval process, you have to do more than just follow a checklist—you need to visualize the workflow. If you treat the MAA as a static document, you risk missing the "invisible" connections between departments that can cause bottlenecks and delays. This is where Business Process Model and Notation (BPMN) serves its purpose.
BPMN is a standardized method for visualizing complex workflows. It provides a clear, standardized visual language that can turn an abstract regulatory manual into a living, breathing roadmap, making it an essential tool for mastering the EMA drug approval process.
By using BPMN, you can:
- Map every decision point: know exactly who handles what and when, from the first submission to the final Commission decision.
- Identify bottlenecks: see where document preparation might slow down or where a "clock stop" requires a surge in resources.
- Bridge the gap: help stakeholders from R&D, legal, and clinical teams speak the same language using a universal standard.
In the high-stakes world of regulatory affairs in pharma, clarity equals speed. Modeling your process in Cardanit helps you transform your workflow, moving from the current scenario “as is” to the future improved process “to be,” while ensuring total traceability across the entire lifecycle.
Key benefits of MAA process mapping with BPMN
Why should pharma regulatory affairs teams switch to BPMN mapping? Here are the facts:
- Standardization: use consistent symbols so everyone—from your internal team to external consultants—understands the map immediately.
- Total visibility: see the end-to-end flow. This makes it easy to spot non-value-added activities or potential compliance gaps before they become troublesome.
- Team alignment: when you align on the process scope before execution, you drastically reduce rework and late-stage delays.
- Predictability: Cardanit BPMN allows you to simulate workflows. You can test "what-if" scenarios regarding resource allocation or how a delay in one lane affects the final submission date.
- Audit readiness: in drug regulatory affairs, if it isn’t documented, it didn’t happen. BPMN ensures every step is traceable, compliant, and ready for scrutiny.
The practical guide: designing and simulating the workflow
To help you visualize the MAA Process in lanes, diagrams and tasks and introduce you to Cardanit BPMN modeling and simulation features, we’ve mapped the high-level flow of the EMA approval process. You can take it from here and structure all the subprocesses related to specific procedural activities of specific stakeholders.
Ready to start? Here’s how you can design a robust MAA workflow using Cardanit.
1. Define the Pool and Lanes
Start by identifying the actors. In an MAA process, you’ll likely have lanes for the Applicant (your company), the EMA/Rapporteurs, and the European Commission. In general, lanes are used in BPMN visual representation to create clear accountability for every task and prevent "lost" communications.
2. Map the milestones
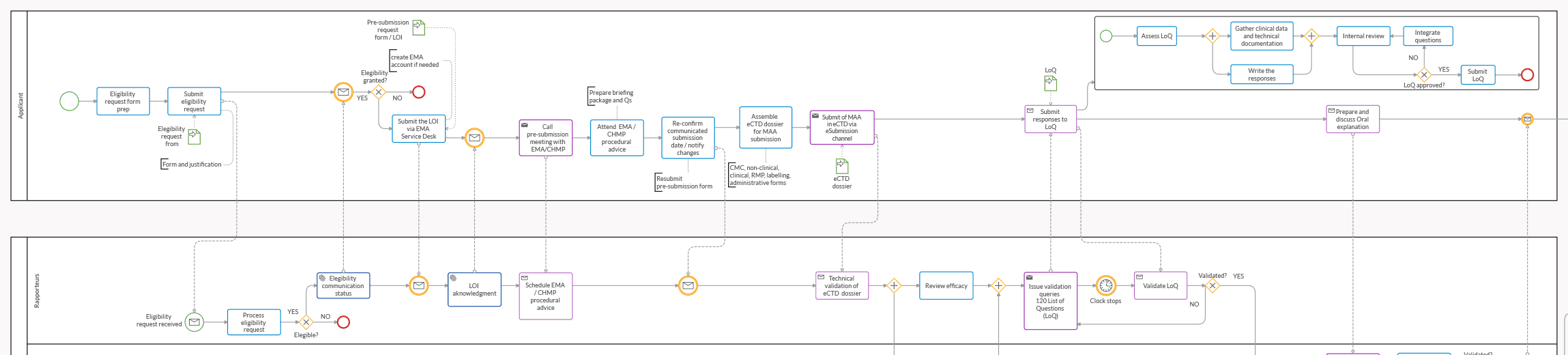
Front-load the important stages of the EMA drug approval process. Include the initial submission, the validation phase, and the critical evaluation phases. Don't forget to model the "Clock Stop" after Day 120—this is a vital part of the pharmaceutical drug regulatory affairs timeline.
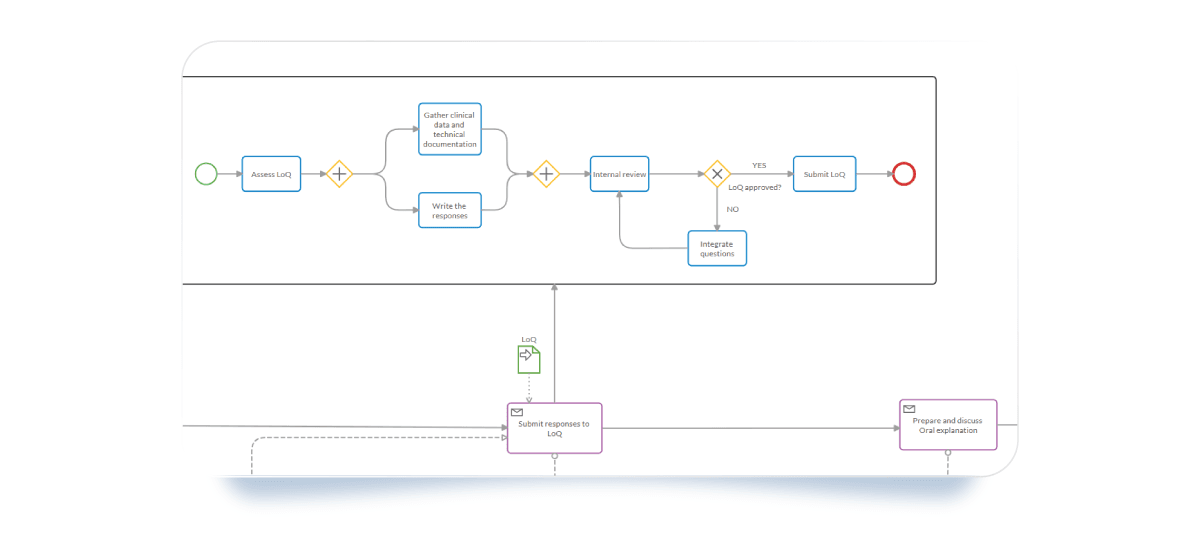
3. Use the Subprocess for the day 120 phase
The Day 120 phase is perhaps the most critical moment in the timeline. Your team must address the List of Questions (LoQ) with absolute precision to keep the process moving. We represented it with these specific elements to manage it:
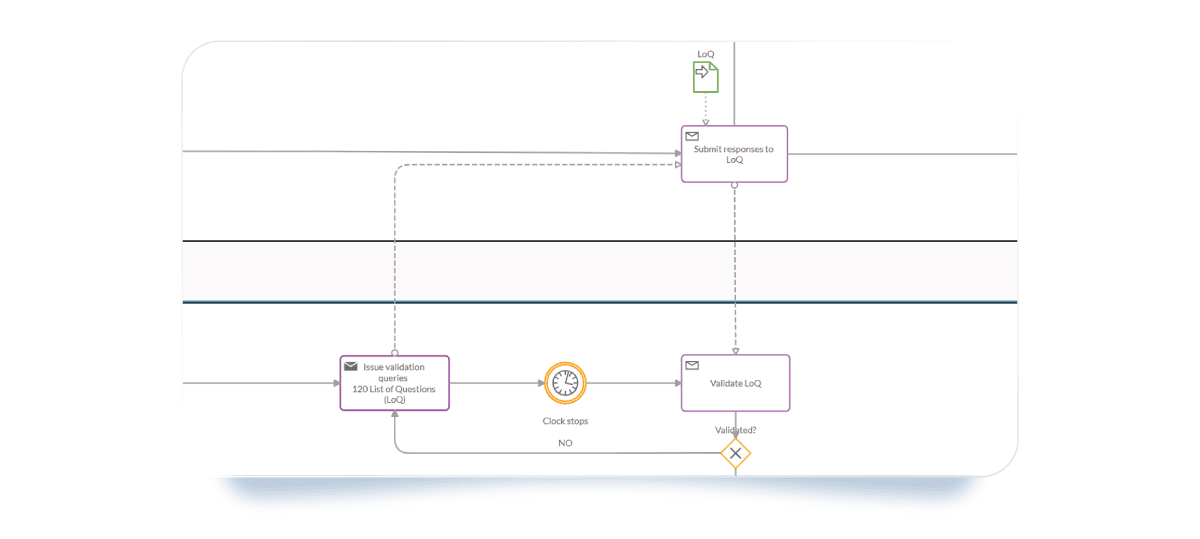
- Intermediate Catch Event (Timer): This represents the "Clock Stop." It keeps the countdown (usually 3 to 6 months) visible to the whole team.
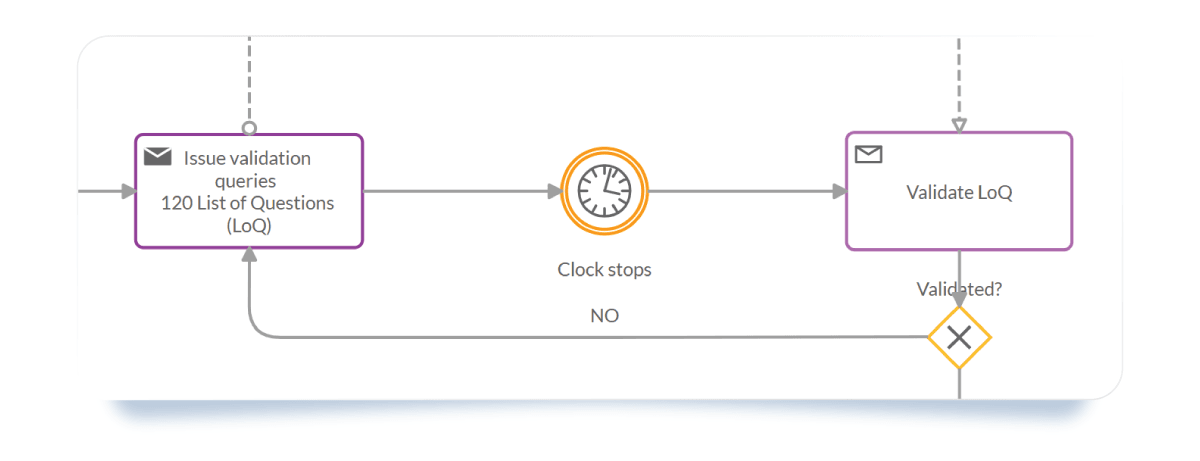
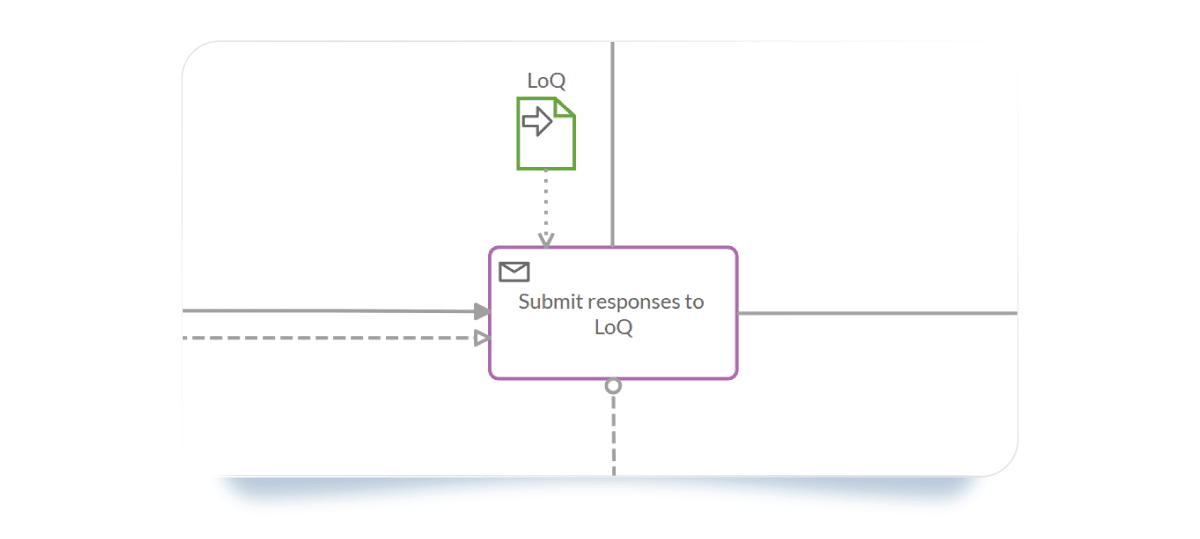
- Data Objects: Link specific documents like the "Response to the List of Questions" to specific tasks to ensure the right version is always used.
- Exclusive Gateway: Create an internal review loop. Does the response meet all EMA requirements? If not, the loop ensures it goes back for drafting before the final submission.
- Parallel Gateway: Use it to represent tasks occurring concurrently but independently from one another. It breaks a flow into parallel paths and ensures the process only continues once all parallel activities are finalized.
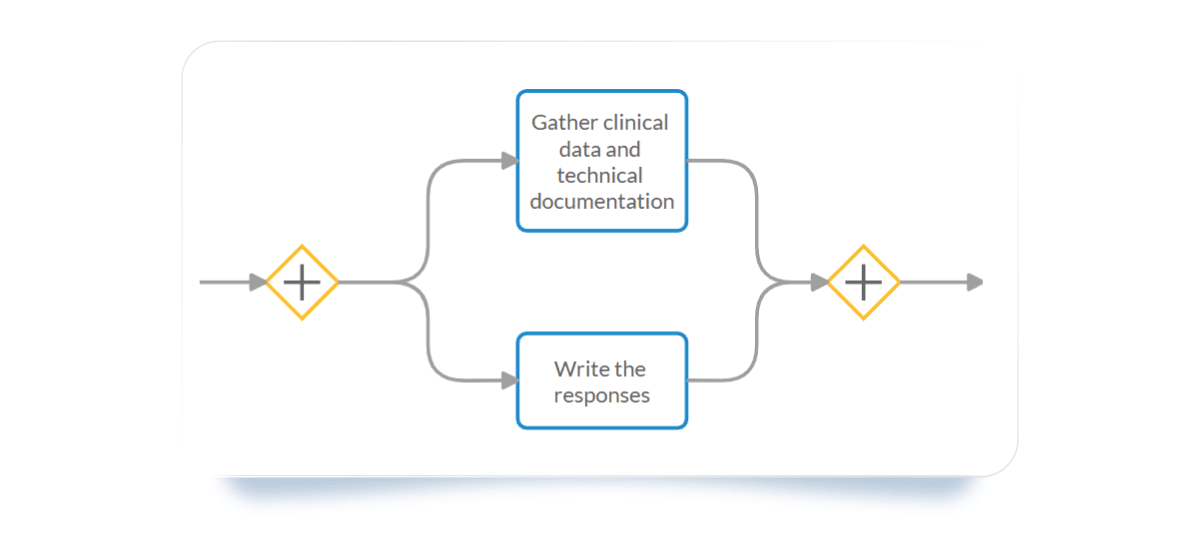
- Subprocess: Use it to represent your team’s tasks that are specific to the Response to the List of Questions. It’s embedded within the Process and helps simplify complex models by organizing work into manageable units.
| BPMN element | Role in MAA process | Benefit for Regulatory Affairs |
|---|---|---|
| Pools & Lanes | Define roles (Applicant, EMA/Rapporteurs,Scientific Committee, European Commission). | Ensures clear accountability and ownership. |
| Intermediate Catch Event | Models "Clock Stops" and legal deadlines. | Keeps the team focused on strict EMA timelines. |
| Data Object | Represents the eCTD or specific documents, such as 120 List of Questions (LoQ). | Ensures document traceability and audit readiness. |
| Exclusive Gateway | Internal quality gates and review loops. | Reduces rework by catching errors before submission. |
| Parallel Gateway | Enables multiple activities to proceed simultaneously. | Improves transparency across tasks. |
| Subprocess | Groups related activities together. | Represents specific subtasks for more manageable workflows. |
4. Simulate real-case scenarios before process implementation
Before you go live, use Cardanit features to check your process logic. Is there a dead-end? Cumbersome tasks? Unclear accountabilities? Simulation helps you catch these errors early. Once your logic is sound, you can simulate the workflow to see how different resource levels or “Clock Stop” scenarios affect your LoQ submission timeline.
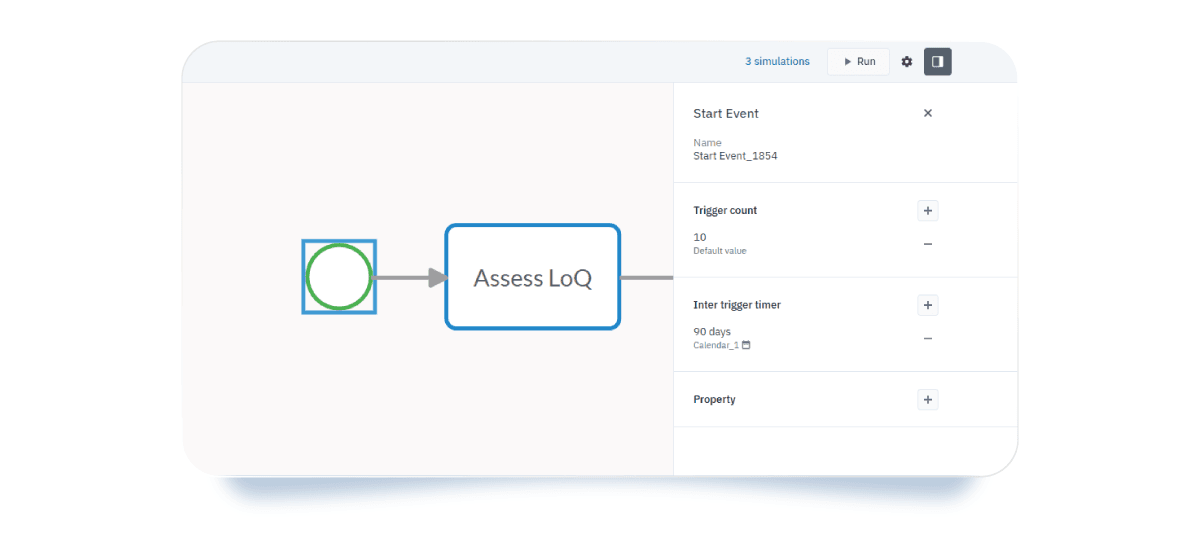
Let’s zoom into a sub-process and use it as a simulation example. To start your simulation, simply switch from the Design tab to the Simulation tab and add your BPSim parameters. You can define:
- Trigger time for the start event: set how many times the process can be triggered and the time interval between each occurrence. For example, if you handle 10 drug approvals a year, you can set an interval of 90 days between each LoQ submission.
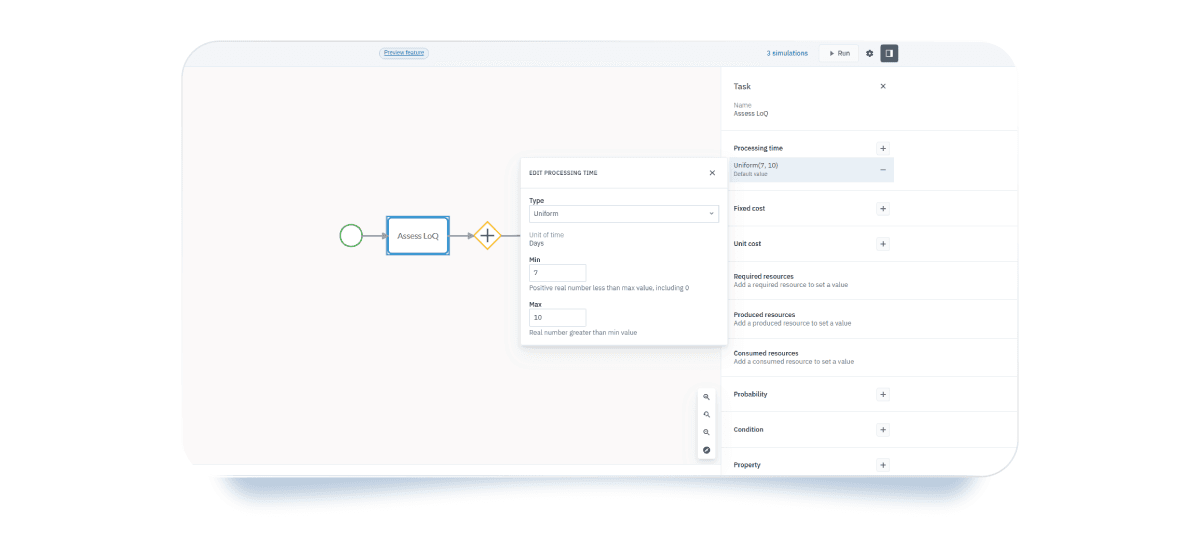
- Task-level parameters: for each task, you can assign different parameters.
- Processing time: Set the average time each task takes to complete. This is especially useful for estimating task duration within a defined time frame, as in your case. When assigning processing time to the LoQ assessment task, we used a uniform distribution to simulate the effort required to assess the List of Questions—between a minimum of 7 days and a maximum of 10 days.
- Calendars: When tasks follow a fixed schedule—such as a 2–3 month timeframe, as in your case—you can define calendar dates. For instance, if you aim to complete the LoQ preparation between February 2 and April 2, you can select those dates in the calendar and then link the calendar to the trigger time.
- Costs: You can assign costs to track monetary expenses related to task execution. In this scenario, it’s advisable to use fixed costs tied to resource expenses. In our example, no costs were assigned.
- Exclusive gateway probabilities: You can estimate the probability of each decision outcome, such as positive or negative paths.
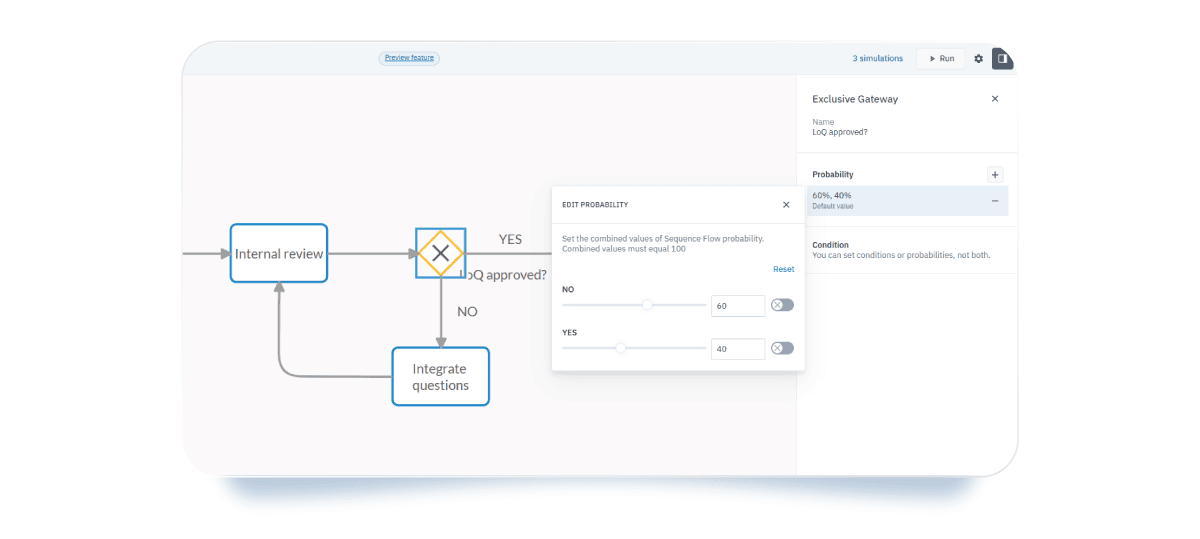
After you’ve configured the simulation properties, run the simulation and see how everything comes together through Cardanit’s data visualization tools. These tools convert raw numbers into clear, easy-to-understand graphics for all stakeholders.
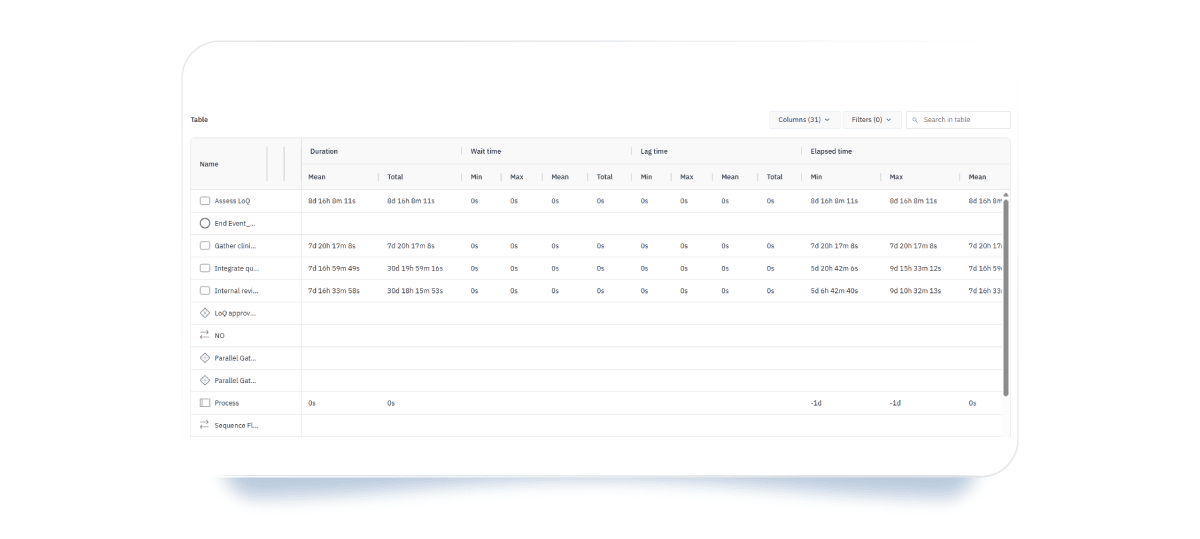
- Tables: present numeric results across rows and columns for all outputs, including tokens, task processing time and duration, costs, and resources. You can filter and sort through activities and data to visualize the insights you’re most interested in.
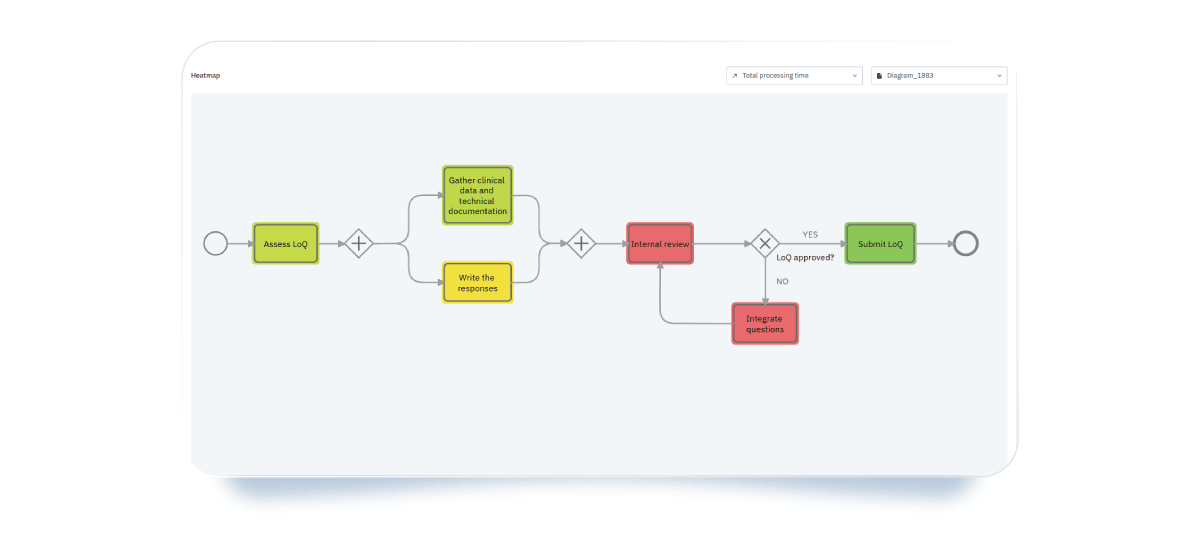
- Heatmaps: the most representative visualization tool, as they highlight bottlenecks—such as long processing times, as shown in this example—using a color scale from hot red to cool green applied directly to activities.
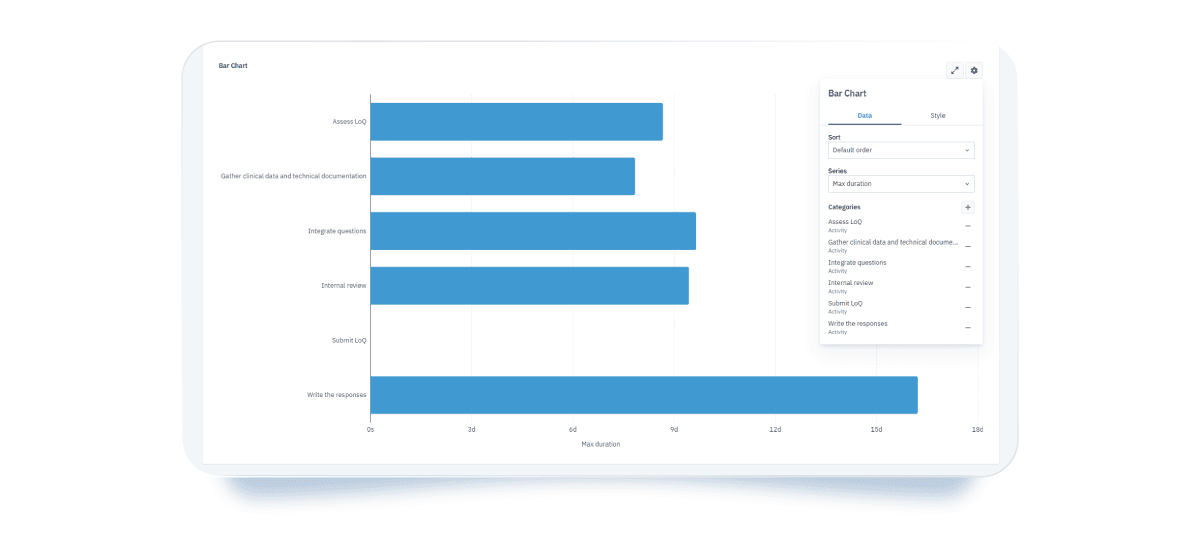
- Bar charts: provide a clear comparison of how BPMN elements perform across selected parameters and help you easily identify bottlenecks. In your case, a bar chart helps highlight tasks with minimum and maximum completion times, supporting better resource allocation and allowing you to structure your process more efficiently.
The improvement process doesn’t stop here. After running the first simulation of your as-is process and analyzing the results, you can identify improvement opportunities and test alternative scenarios within the EMA drug approval process. This allows you to progress from your current “as-is” scenario to a streamlined “to-be” process.
These improvements can include:
- Redesigning the overall workflow to streamline activities
- Reallocating resources to reduce processing time
- Adjusting parameters to optimize overall outcomes
These are key advantages of Business Process Simulation in Cardanit. Cardanit BPMN allows you to stress-test your current process and explore the impact of potential improvements before implementation. This is an essential capability when managing the complexity of the EMA drug approval process.
Simulation reduces the risk of ineffective changes through data-driven decision-making. It also bridges the gap between planning and execution, ensuring your processes are aligned with organizational goals and ready for deployment in pharmaceutical drug regulatory affairs.
Plus, sharing your projects with stakeholders is only a click away. With real-time process alignment, you always know who is involved in each stage of the process, where the workflow stands, and where the relevant documents are.
Summary
The EMA drug approval process is complex, but it doesn't have to be a black box. By using BPMN to map your MAA journey, you turn a fragmented procedure into a streamlined, detailed workflow. You gain more than just a diagram; you gain a roadmap to regulatory success that keeps your team aligned and your submission on track.
In a nutshell, the key advantages of using BPMN in regulatory affairs are:
- Workflow clarity and standardization: Mapping processes clarifies roles, tasks, and timelines.
- Improved cross-team collaboration: Alignment across teams reduces miscommunication and delays.
- Data-driven optimization: Workflow simulation enables data-based process improvements.
- Traceability and compliance: All tasks, documents, and decisions are fully documented and audit-ready
At Cardanit, we’re always looking for ways to make complex architectures easier to manage. Whether you’re a pro player in regulatory affairs or just starting your first submission, Cardanit BPMN gives you the precision and compliance you need to succeed.
Andrea is the collective pseudonym for the group of people working behind Cardanit, the Business Process Management Software as a Service of ESTECO. The group has different backgrounds and several decades of experience in fields varying from BPM, BPMN, DMN, Process Mining, Simulation, Optimization, Numerical Methods, Research and Development, and Marketing.
Andrea is the collective pseudonym for the group of people working behind Cardanit, the Business Process Management Software as a Service of ESTECO. The group has different backgrounds and several decades of experience in fields varying from BPM, BPMN, DMN, Process Mining, Simulation, Optimization, Numerical Methods, Research and Development, and Marketing.
People also ask
BPMN 2.0 is the global standard for process modeling, offering rich, standardized diagrams that provide the precision required for technical audits and legal compliance. This universal language ensures that regulatory requirements are easily mapped and auditable. Unlike simple flowcharts, its XML-based structure also enables automated verification to guarantee that all regulatory mandates are met.
DMN complements BPMN by separating decision logic from the process flow, keeping diagrams clean and manageable. In Cardanit, DMN decision tables link to BPMN Business Rule Tasks to provide a transparent, precise record of regulatory decision-making.
Choose Cardanit for its intuitive interface and real-time collaboration feature that requires no installation. Cardanit expertly balances ease of use with full BPMN 2.0 compliance and BPSim capabilities for stress-testing critical processes. Try it now.
A business is only as efficient as its processes. What are you waiting to improve yours?
